Why is it difficult to date sedimentary rocks using radiometric dating techniques?
According to plate tectonic theory, continental crust overrides oceanic crust when these plates collide because the continental crust is less dense than the ocean floor. As the ocean floor sinks, it encounters increasing pressures and temperatures within the crust. Ultimately, the pressures and temperatures are so high that the rocks in the subducted oceanic crust melt. Once the rocks melt, a plume of molten material begins to rise in the crust. As the plume rises it melts and incorporates other crustal rocks. This rising body of magma is an open system with respect to the surrounding crustal rocks.
It is possible that these physical processes have an impact on the determined radiometric age of the rock as it cools and crystallizes. Time is not a direct measurement. The actual data are the ratios of parent and daughter isotopes present in the sample. Time is one of the values that can be determined from the slope of the line representing the distribution of the isotopes.
Isotope distributions are determined by the chemical and physical factors governing a given magma chamber.
Navigation menu
Rhyolites in Yellowstone N. Most genetic models for uranium deposits in sandstones in the U. Most of the uranium deposits in Wyoming are formed from uraniferous groundwaters derived from Precambrian granitic terranes. Uranium in the major uranium deposits in the San Juan basin of New Mexico is believed to have been derived from silicic volcanic ash from Jurassic island arcs at the edge of the continent. From the above sources, we see that another factor influencing radiometric dates is the proportion of the magma that comes from subducted oceanic plates and the proportion that comes from crustal rock.
Initially, we would expect most of it to come from subducted oceanic plates, which are uranium and thorium poor and maybe lead rich. Later, more of the crustal rock would be incorporated by melting into the magma, and thus the magma would be richer in uranium and thorium and poorer in lead. So this factor would also make the age appear to become younger with time. There are two kinds of magma, and the crustal material which is enriched in uranium also tends to be lighter.
For our topic on radiometric dating and fractional crystallization, there is nothing that would prevent uranium and thorium ores from crystallizing within the upper, lighter portion of the magma chamber and descending to the lower boundaries of the sialic portion. The upper portion of the sialic magma would be cooler since its in contact with continental rock, and the high melting point of UO sub 2 uranium dioxide, the common form in granite: The same kind of fractional crystallization would be true of non-granitic melts.
I think we can build a strong case for fictitious ages in magmatic rocks as a result of fractional cystallization and geochemical processes.
Why is it difficult to date sedimentary rocks using radiometric dating techniques? | Socratic
As we have seen, we cannot ignore geochemical effects while we consider geophysical effects. Sialic granitic and mafic basaltic magma are separated from each other, with uranium and thorium chemically predestined to reside mainly in sialic magma and less in mafic rock. Here is yet another mechanism that can cause trouble for radiometric dating: As lava rises through the crust, it will heat up surrounding rock.
Lead has a low melting point, so it will melt early and enter the magma. This will cause an apparent large age.
Uranium has a much higher melting point. It will enter later, probably due to melting of materials in which it is embedded. This will tend to lower the ages. Mechanisms that can create isochrons giving meaningless ages: Geologists attempt to estimate the initial concentration of daughter product by a clever device called an isochron. Let me make some general comments about isochrons. The idea of isochrons is that one has a parent element, P, a daughter element, D, and another isotope, N, of the daughter that is not generated by decay.
One would assume that initially, the concentration of N and D in different locations are proportional, since their chemical properties are very similar. Note that this assumption implies a thorough mixing and melting of the magma, which would also mix in the parent substances as well. Then we require some process to preferentially concentrate the parent substances in certain places. Radioactive decay would generate a concentration of D proportional to P. By taking enough measurements of the concentrations of P, D, and N, we can solve for c1 and c2, and from c1 we can determine the radiometric age of the sample.
Otherwise, the system is degenerate. Thus we need to have an uneven distribution of D relative to N at the start. If these ratios are observed to obey such a linear relationship in a series of rocks, then an age can be computed from them. The bigger c1 is, the older the rock is. That is, the more daughter product relative to parent product, the greater the age. Thus we have the same general situation as with simiple parent-to-daughter computations, more daughter product implies an older age.
This is a very clever idea. However, there are some problems with it. First, in order to have a meaningful isochron, it is necessary to have an unusual chain of events. Initially, one has to have a uniform ratio of lead isotopes in the magma.
Search form
Usually the concentration of uranium and thorium varies in different places in rock. This will, over the assumed millions of years, produce uneven concentrations of lead isotopes. To even this out, one has to have a thorough mixing of the magma. Even this is problematical, unless the magma is very hot, and no external material enters. Now, after the magma is thoroughly mixed, the uranium and thorium will also be thoroughly mixed.
What has to happen next to get an isochron is that the uranium or thorium has to concentrate relative to the lead isotopes, more in some places than others. So this implies some kind of chemical fractionation.
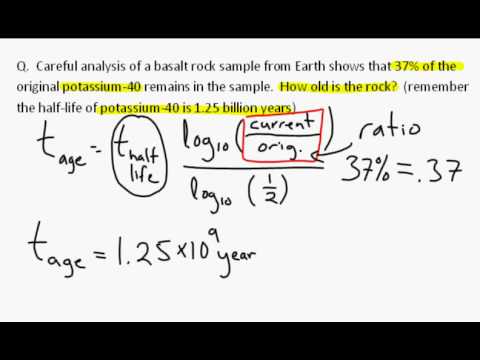
Then the system has to remain closed for a long time. This chemical fractionation will most likely arise by some minerals incorporating more or less uranium or thorium relative to lead.
Radiometric dating
Anyway, to me it seems unlikely that this chain of events would occur. Another problem with isochrons is that they can occur by mixing and other processes that result in isochrons yielding meaningless ages. Sometimes, according to Faure, what seems to be an isochron is actually a mixing line, a leftover from differentiation in the magma. Fractionation followed by mixing can create isochrons giving too old ages, without any fractionation of daughter isotopes taking place.
To get an isochron with a false age, all you need is 1 too much daughter element, due to some kind of fractionation and 2 mixing of this with something else that fractionated differently. Since fractionation and mixing are so common, we should expect to find isochrons often. How they correlate with the expected ages of their geologic period is an interesting question.
There are at least some outstanding anomalies. Faure states that chemical fractionation produces "fictitious isochrons whose slopes have no time significance. As an example, he uses Pliocene to Recent lava flows and from lava flows in historical times to illustrate the problem. He says, these flows should have slopes approaching zero less than 1 million years , but they instead appear to be much older million years.
Steve Austin has found lava rocks on the Uinkeret Plateau at Grand Canyon with fictitious isochrons dating at 1. Then a mixing of A and B will have the same fixed concentration of N everywhere, but the amount of D will be proportional to the amount of P. This produces an isochron yielding the same age as sample A. This is a reasonable scenario, since N is a non-radiogenic isotope not produced by decay such as lead , and it can be assumed to have similar concentrations in many magmas. Magma from the ocean floor has little U and little U and probably little lead byproducts lead and lead Magma from melted continental material probably has more of both U and U and lead and lead Thus we can get an isochron by mixing, that has the age of the younger-looking continental crust.
The age will not even depend on how much crust is incorporated, as long as it is non-zero. However, if the crust is enriched in lead or impoverished in uranium before the mixing, then the age of the isochron will be increased.
