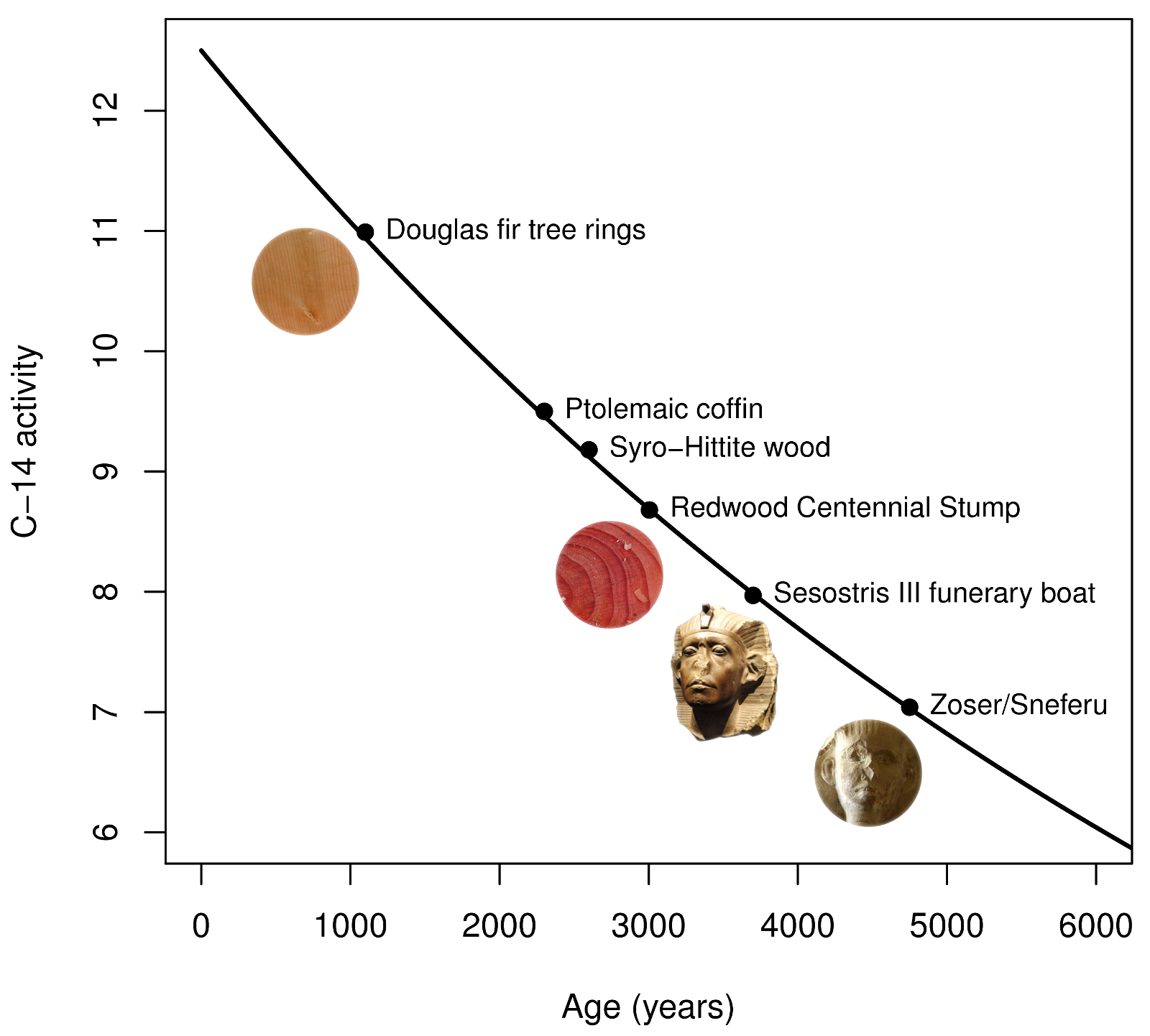
Once the organism dies then they stop breathing and the carbon in the remains decays. Finding the remains we work out the fraction of radioactive carbon and deduce the time since it died.
Related Questions How does radioactive decay relate to radiometric dating? Do creationists have a clue about radiometric dating? What is the dating method based on rate of decay of radioactive isotopes? Can radiometric dating be used to find the absolute age of any material? How do you date fossils? Answer Questions Three forces acting on a spherical mass M are depicted in the diagram below.? Pls help with this physics question? The carbon ends up as a trace component in atmospheric carbon dioxide CO 2.
Radiometric dating
A carbon-based life form acquires carbon during its lifetime. Plants acquire it through photosynthesis , and animals acquire it from consumption of plants and other animals. When an organism dies, it ceases to take in new carbon, and the existing isotope decays with a characteristic half-life years. The proportion of carbon left when the remains of the organism are examined provides an indication of the time elapsed since its death.
This makes carbon an ideal dating method to date the age of bones or the remains of an organism. The carbon dating limit lies around 58, to 62, years. The rate of creation of carbon appears to be roughly constant, as cross-checks of carbon dating with other dating methods show it gives consistent results.
However, local eruptions of volcanoes or other events that give off large amounts of carbon dioxide can reduce local concentrations of carbon and give inaccurate dates. The releases of carbon dioxide into the biosphere as a consequence of industrialization have also depressed the proportion of carbon by a few percent; conversely, the amount of carbon was increased by above-ground nuclear bomb tests that were conducted into the early s.
Radiometric dating - Wikipedia
Also, an increase in the solar wind or the Earth's magnetic field above the current value would depress the amount of carbon created in the atmosphere. This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons. This causes induced fission of U, as opposed to the spontaneous fission of U.
The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the neutron flux. This scheme has application over a wide range of geologic dates. For dates up to a few million years micas , tektites glass fragments from volcanic eruptions , and meteorites are best used.
Older materials can be dated using zircon , apatite , titanite , epidote and garnet which have a variable amount of uranium content. The technique has potential applications for detailing the thermal history of a deposit. The residence time of 36 Cl in the atmosphere is about 1 week. Thus, as an event marker of s water in soil and ground water, 36 Cl is also useful for dating waters less than 50 years before the present.
Luminescence dating methods are not radiometric dating methods in that they do not rely on abundances of isotopes to calculate age. Instead, they are a consequence of background radiation on certain minerals. Over time, ionizing radiation is absorbed by mineral grains in sediments and archaeological materials such as quartz and potassium feldspar.
- How is radioactive decay related to radiometric dating? | Yahoo Answers.
- how to see if my husband is on dating sites!
- free dating open source php!
The radiation causes charge to remain within the grains in structurally unstable "electron traps". Exposure to sunlight or heat releases these charges, effectively "bleaching" the sample and resetting the clock to zero.
The trapped charge accumulates over time at a rate determined by the amount of background radiation at the location where the sample was buried. Stimulating these mineral grains using either light optically stimulated luminescence or infrared stimulated luminescence dating or heat thermoluminescence dating causes a luminescence signal to be emitted as the stored unstable electron energy is released, the intensity of which varies depending on the amount of radiation absorbed during burial and specific properties of the mineral.
These methods can be used to date the age of a sediment layer, as layers deposited on top would prevent the grains from being "bleached" and reset by sunlight. Pottery shards can be dated to the last time they experienced significant heat, generally when they were fired in a kiln. Absolute radiometric dating requires a measurable fraction of parent nucleus to remain in the sample rock. For rocks dating back to the beginning of the solar system, this requires extremely long-lived parent isotopes, making measurement of such rocks' exact ages imprecise.
To be able to distinguish the relative ages of rocks from such old material, and to get a better time resolution than that available from long-lived isotopes, short-lived isotopes that are no longer present in the rock can be used. At the beginning of the solar system, there were several relatively short-lived radionuclides like 26 Al, 60 Fe, 53 Mn, and I present within the solar nebula.
These radionuclides—possibly produced by the explosion of a supernova—are extinct today, but their decay products can be detected in very old material, such as that which constitutes meteorites. By measuring the decay products of extinct radionuclides with a mass spectrometer and using isochronplots, it is possible to determine relative ages of different events in the early history of the solar system. Dating methods based on extinct radionuclides can also be calibrated with the U-Pb method to give absolute ages. Thus both the approximate age and a high time resolution can be obtained.
Generally a shorter half-life leads to a higher time resolution at the expense of timescale. The iodine-xenon chronometer [32] is an isochron technique. Samples are exposed to neutrons in a nuclear reactor. This converts the only stable isotope of iodine I into Xe via neutron capture followed by beta decay of I.
Categories
After irradiation, samples are heated in a series of steps and the xenon isotopic signature of the gas evolved in each step is analysed. Samples of a meteorite called Shallowater are usually included in the irradiation to monitor the conversion efficiency from I to Xe. This in turn corresponds to a difference in age of closure in the early solar system.
Another example of short-lived extinct radionuclide dating is the 26 Al — 26 Mg chronometer, which can be used to estimate the relative ages of chondrules. The 26 Al — 26 Mg chronometer gives an estimate of the time period for formation of primitive meteorites of only a few million years 1. From Wikipedia, the free encyclopedia.
Earth sciences portal Geophysics portal Physics portal. The disintegration products of uranium". American Journal of Science. Radiometric Dating and the Geological Time Scale: Circular Reasoning or Reliable Tools? In Roth, Etienne; Poty, Bernard. Nuclear Methods of Dating. Radiometric dating is the technique of using isotopic ratios of common elements to determine the age approximate of materials associated with the element, such as trees, rock strata, fossils, human artifacts and the like.
- online dating articles new york times!
- Navigation menu.
- dating factory make money!
Because radioactive decay follows a specific mathematical formula and is dependent upon unique decay rates half-lives of each element, formulas can be developed that compare the measured amounts of different elemental isotopes. The ratio of the difference is related mathematically to the time that is took for the original sample to decay into the ratios found.
How does radioactive decay relate to radiometric dating? That is the time duration that is used for radiometric dating.
