This means that as the rubidium decays and more strontium is formed, the ratio will change. The half-life of rubidium is Uranium-lead dating is one of the most complicated of all dating techniques. This is in part because uranium and lead are not retained in rocks as easily as some others, and in part because the parent isotopes and daughter products are not even directly related.
- Radiometric Dating.
- Radiometric Dating: Methods, Uses & the Significance of Half-Life?
- ?
- dating games for ipad free;
- etiquette dating in japan;
For the isotopes uranium and uranium to respectively become lead and lead, they must first undergo a serious of highly unstable transformations into isotopes with very short half-lives. However, if one knows the scientific formula for interpreting these transitions, the results can be "highly precise" according to paleontologist Guy Narbonne Kerr, The half-life of uranium is million years, while the half-life of uranium is 4. This website is part of a Geology class project on Processes in Physical Geology.
You must create an account to continue watching
Revised 25 February Send corrections or comments to parkero earlham. Origins of Radiometric Dating. Types of Radiometric Dating. Common Types of Radiometric Dating Carbon 14 Dating As shown in the diagram above, the radioactive isotope carbon originates in the Earth's atmosphere, is distributed among the living organisms on the surface, and ceases to replenish itself within an organism after that organism is dead.
Potassium-Argon Dating The isotope potassium k decays into a fixed ratio of calcium and argon This method for rock dating is based on the decay of potassium into argon: One problem is that potassium is also highly mobile and may move into older rocks. This depends on the decay of uranium and uranium to isotopes of lead.
Due to the long half-life of uranium it is not suitable for short time periods, such as most archaeological purposes, but it can date the oldest rocks on earth. A important limitation of radiometric dating often overlooked by layman and not always made clear in scholarly works as well is that any date is actually a range, following the 68—95— This leaves out important information which would tell you how precise is the dating result.
Description
Carbon dating has an interesting limitation in that the ratio of regular carbon to carbon in the air is not constant and therefore any date must be calibrated using dendrochronology. Another limitation is that carbon can only tell you when something was last alive, not when it was used.
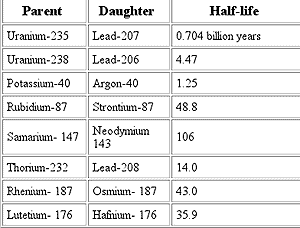
A limitation with all forms of radiometric dating is that they depend on the presence of certain elements in the substance to be dated. Carbon dating works on organic matter, all of which contains carbon. However it is less useful for dating metal or other inorganic objects. Most rocks contain uranium, allowing uranium-lead and similar methods to date them.
- can you feel it lyrics chinese dating show;
- dating comedy chicago;
- dating site for overweight singles;
- Navigation menu.
Other elements used for dating, such as rubidium, occur in some minerals but not others, restricting usefulness. Note that although carbon dating receives a lot of attention, since it can give information about the relatively recent past, it is rarely used in geology and almost never used to date fossils. Carbon decays almost completely within , years of the organism dying, and many fossils and rock strata are hundreds of times older than that. To date older fossils, other methods are used, such as potassium-argon or argon-argon dating.
Other forms of dating based on reactive minerals like rubidium or potassium can date older finds including fossils, but have the limitation that it is easy for ions to move into rocks post-formation so that care must be taken to consider geology and other factors. Radiometric dating — through processes similar to those outlined in the example problem above — frequently reveals that rocks, fossils , etc.
The oldest rock so far dated is a zircon crystal that formed 4. They tie themselves in logical knots trying to reconcile the results of radiometric dating with the unwavering belief that the Earth was created ex nihilo about 6, to 10, years ago. Creationists often blame contamination.
Earlham College - Geology - Radiometric Dating
Indeed, special creationists have for many years held that where science and their religion conflict, it is a matter of science having to catch up with scripture, not the other way around. One way Young Earth Creationists and other denialists try to discredit radiometric dating is to cite examples radiometric dating techniques providing inaccurate results.
This is frequently because the selected technique is used outside of its appropriate range, for example on very recent lavas. In attempting to date Mt. Helens, creationists attempted discredit the discipline through dishonest practices. Ultimately these "creation scientists" were forced to admit that even for methods they accepted as sound, the age of the Earth would be vastly greater than the 6, they set out to prove.
Radiometric dating
Creationists commonly object to carbon dating results on the basis that they can be contaminated in the laboratory by atmospheric carbon; however such contamination would result in increased carbon levels and hence the object appearing younger than it is; hence samples can only be older than they appear, not younger, which does not help young earth creationists at all. Another creationist argument is to claim that rates of atomic decay are not constant through time.
An enormous amount of research shows that in the lab decay rates are constant over time and wherever you are. Faced with this, creationists say that you can't extrapolate from this to deduce they are correct over billions of years. A few experiments have found small variations in decay rates, at least for some forms of decay and some isotopes.
While it may require further investigation to see if this is a real phenomenon, even the biggest positive results do not offer anything like a variation that would allow the truth of young earth creationism.
Not to be confused with single's night for devilish ham radio enthusiasts. See the main article on this topic: We are to teach what the Bible says and let scientific research and discovery catch up to the truth of Scripture. Science is not a priority tool of biblical interpretation.
