Alpha radiation can be stopped by paper, beta radiation can be stopped by wood, while gamma radiation is stopped by lead. Types of Radioactive Decay. Radioisotopes decay at a constant rate and the time taken for half the original radioisotope to decay is known as the half life. While radioisotope dating is the most commonly used method for dating fossils, other techniques do exist. As the isotopes decay, they give off particles from their nucleus and become a different isotope.
The parent isotope is the original unstable isotope, and daughter isotopes are the stable product of the decay.
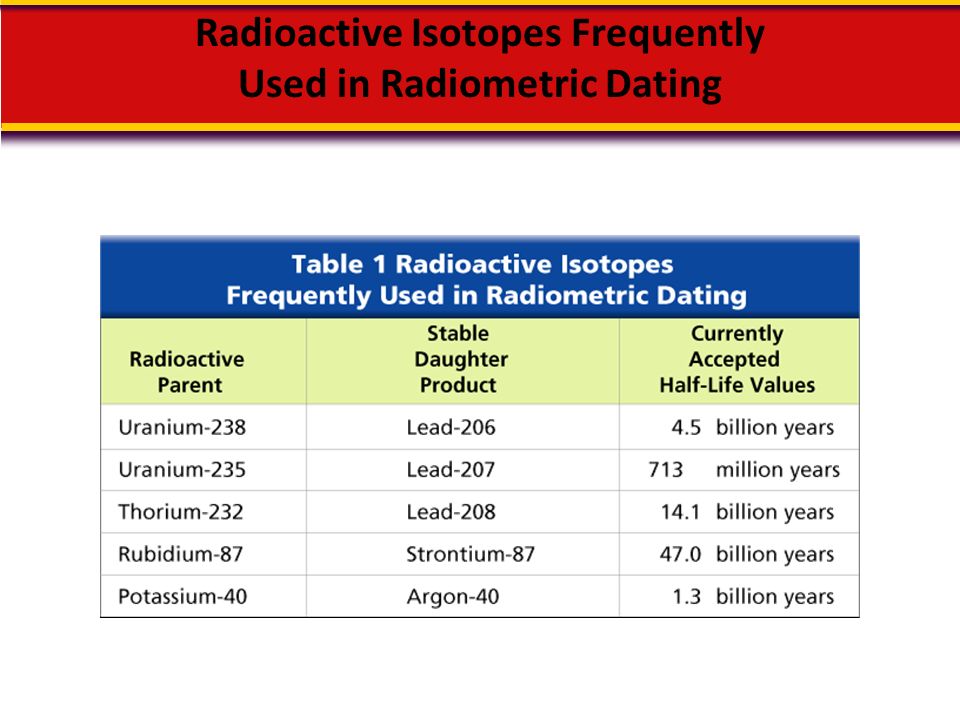
Radioactive Decay
Half-life is the amount of time it takes for half of the parent isotopes to decay. The decay occurs on a logarithmic scale. For example, the half-life of C is 5, years. In the first 5, years, the organism will lose half of its C isotopes. In another 5, years, the organism will lose another half of the remaining C isotopes. This process continues over time, with the organism losing half of the remaining C isotopes each 5, years. Fossils are collected along with rocks that occur from the same strata. These samples are carefully cataloged and analyzed with a mass spectrometer.
You must create an account to continue watching
The mass spectrometer is able to give information about the type and amount of isotopes found in the rock. Scientists find the ratio of parent isotope to daughter isotope. By comparing this ratio to the half-life logarithmic scale of the parent isotope, they are able to find the age of the rock or fossil in question. The radiation causes charge to remain within the grains in structurally unstable "electron traps". Exposure to sunlight or heat releases these charges, effectively "bleaching" the sample and resetting the clock to zero.
The trapped charge accumulates over time at a rate determined by the amount of background radiation at the location where the sample was buried. Stimulating these mineral grains using either light optically stimulated luminescence or infrared stimulated luminescence dating or heat thermoluminescence dating causes a luminescence signal to be emitted as the stored unstable electron energy is released, the intensity of which varies depending on the amount of radiation absorbed during burial and specific properties of the mineral.
These methods can be used to date the age of a sediment layer, as layers deposited on top would prevent the grains from being "bleached" and reset by sunlight. Pottery shards can be dated to the last time they experienced significant heat, generally when they were fired in a kiln. Absolute radiometric dating requires a measurable fraction of parent nucleus to remain in the sample rock. For rocks dating back to the beginning of the solar system, this requires extremely long-lived parent isotopes, making measurement of such rocks' exact ages imprecise.
To be able to distinguish the relative ages of rocks from such old material, and to get a better time resolution than that available from long-lived isotopes, short-lived isotopes that are no longer present in the rock can be used. At the beginning of the solar system, there were several relatively short-lived radionuclides like 26 Al, 60 Fe, 53 Mn, and I present within the solar nebula. These radionuclides—possibly produced by the explosion of a supernova—are extinct today, but their decay products can be detected in very old material, such as that which constitutes meteorites.
By measuring the decay products of extinct radionuclides with a mass spectrometer and using isochronplots, it is possible to determine relative ages of different events in the early history of the solar system. Dating methods based on extinct radionuclides can also be calibrated with the U-Pb method to give absolute ages.
Thus both the approximate age and a high time resolution can be obtained. Generally a shorter half-life leads to a higher time resolution at the expense of timescale. The iodine-xenon chronometer [32] is an isochron technique. Samples are exposed to neutrons in a nuclear reactor. This converts the only stable isotope of iodine I into Xe via neutron capture followed by beta decay of I. After irradiation, samples are heated in a series of steps and the xenon isotopic signature of the gas evolved in each step is analysed.
Samples of a meteorite called Shallowater are usually included in the irradiation to monitor the conversion efficiency from I to Xe. This in turn corresponds to a difference in age of closure in the early solar system. Another example of short-lived extinct radionuclide dating is the 26 Al — 26 Mg chronometer, which can be used to estimate the relative ages of chondrules. The 26 Al — 26 Mg chronometer gives an estimate of the time period for formation of primitive meteorites of only a few million years 1.
From Wikipedia, the free encyclopedia. Earth sciences portal Geophysics portal Physics portal. The disintegration products of uranium". American Journal of Science. Radiometric Dating and the Geological Time Scale: Circular Reasoning or Reliable Tools?
Radiometric dating
In Roth, Etienne; Poty, Bernard. Nuclear Methods of Dating. Annual Review of Nuclear Science. Earth and Planetary Science Letters. The age of the earth. Radiogenic isotope geology 2nd ed. Principles and applications of geochemistry: Englewood Cliffs, New Jersey: United States Geological Survey.
- Radiometric Dating: Methods, Uses & the Significance of Half-Life.
- .
- .
- dating pocket watch cases.
- beautifulpeople online dating.
Journal of African Earth Sciences. South African Journal of Geology. New Tools for Isotopic Analysis". The Swedish National Heritage Board. Archived from the original on 31 March Retrieved 9 March Bispectrum of 14 C data over the last years" PDF.
Radioactive Dating | BioNinja
Planetary Sciences , page Cambridge University Press, Meteoritics and Planetary Science. Canon of Kings Lists of kings Limmu. Chinese Japanese Korean Vietnamese. Lunisolar Solar Lunar Astronomical year numbering. Deep time Geological history of Earth Geological time units. Chronostratigraphy Geochronology Isotope geochemistry Law of superposition Luminescence dating Samarium—neodymium dating.
Amino acid racemisation Archaeomagnetic dating Dendrochronology Ice core Incremental dating Lichenometry Paleomagnetism Radiometric dating Radiocarbon Uranium—lead Potassium—argon Tephrochronology Luminescence dating Thermoluminescence dating. Fluorine absorption Nitrogen dating Obsidian hydration Seriation Stratigraphy. Retrieved from " https: